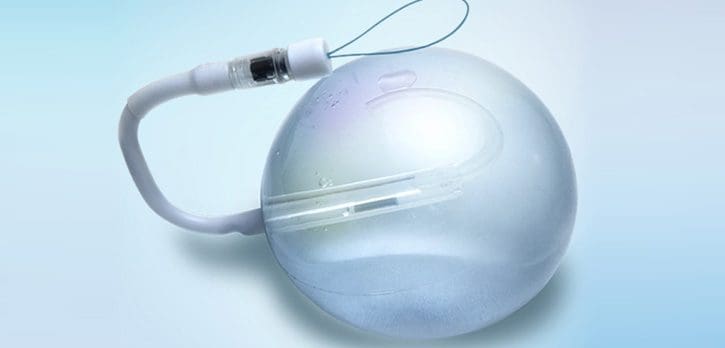
The Spatz gastric balloon has recently been approved by the FDA in the US, and over 100,000 have been implanted outside the US over the last decade. When the FDA approves a medical device, the manufacturer of the device must do a “Post Approval Study” (commonly abbreviated as “PAS”) on their device and submit data on actual patients in whom the device was implanted.
JourneyLite has participated in post approval studies on all gastric balloon systems that have come to market in the US, and we are proud to have been selected to participate in the Spatz PAS.
There are always specific criteria for patient inclusion and exclusion, and in order to be considered for participation in the Spatz PAS a patient must meet the following guidelines:
Inclusion Criteria (you must meet all of these conditions to be considered for the study):
- Age 22-65
- BMI 35-40, or BMI 30-34.9 with one or more major obesity-related conditions (diabetes, hypertension, sleep apnea, etc)
- Willing to comply with substantial lifelong dietary restrictions required by the procedure
- History of obesity (BMI 30+) for at least 2 years
- History of failure with non-surgical weight loss methods
- Willingness to follow protocol requirements, including signed informed consent, routine follow-up schedule, completing laboratory tests, and completing diet counseling
- Residing within a reasonable distance from the investigator’s office and able to travel to the investigator to complete all routine follow-up visits
- Ability to give informed consent
- Women of childbearing potential (i.e. not post-menopausal or surgically sterilized) must agree to use adequate birth control methods. Acceptable methods are limited to hormonal contraceptives (oral, flexible vaginal ring, skin patch, injection), diaphragms, IUDs, condoms with or without spermicide, and voluntary abstinence.
Exclusion Criteria (if you have any of these things you cannot be in the study):
- Prior surgery involving the esophagus, stomach, and duodenum or hiatal hernia surgery
- Prior open or laparoscopic bariatric surgery
- Any inflammatory disease of the gastrointestinal tract including esophagitis, Barrett’s esophagus, gastric ulceration, duodenal ulceration, cancer, or specific inflammation such as Crohn’s disease
- A gastric mass
- A hiatal hernia >2cm or severe or intractable gastro-esophageal reflux symptoms
- Acid reflux symptoms to any degree that require more than one medication for symptom control
- A structural abnormality in the esophagus or pharynx such as a stricture or diverticulum that could impede passage of the balloon alongside the endoscope
- Achalasia or any other severe esophageal motility disorder that may pose a safety risk during the removal of the device
- Severe coagulopathy
- Insulin-dependent diabetes (either Type 1 or Type 2) or a significant likelihood of requiring insulin treatment in the following 12 months
- Subjects with any serious health condition unrelated to their weight that would increase the risk of endoscopy
- Chronic abdominal pain
- Motility disorders of the GI tract such as gross esophageal motility disorders, gastroparesis or intractable constipation
- Hepatic insufficiency or cirrhosis
- Serious or uncontrolled psychiatric illness or disorder that could compromise patient understanding of or compliance with follow up visits and removal of the device after 8 months
- Alcoholism or drug addiction
- Patients unwilling to participate in an established medically-supervised diet and behavior modification program, with routine medical follow-up
- Patients receiving daily prescribed treatment with aspirin, anti-inflammatory agents, anticoagulants or other gastric irritants
- Patients who are unable or unwilling to take prescribed proton pump inhibitor medication for the duration of the device implant
- Patients who are known to have, or suspected to have, an allergic reaction to materials contained in the system
- Patients who have BOTH:
a. A previous history of a serotonin syndrome
AND
b. currently taking any drug known to affect the levels of serotonin in the body [e.g., selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors (MAOIs)] - Patients who are pregnant or breast-feeding
- Subjects with Severe cardiopulmonary disease or other serious organic disease which might include known history of coronary artery disease, Myocardial infarction within the past 6 months, poorly controlled hypertension, required use of NSAIDs
- Subjects who have tested positive for H. Pylori, and who have not yet been treated
If you are interested in the Spatz gastric balloon PAS and you meet the above criteria, the next step in the process would be to schedule a consultation with one of our physicians to discuss the procedure and the study in more detail.

