…
Additional menu
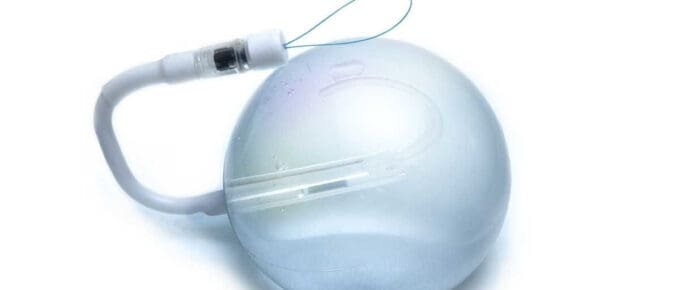
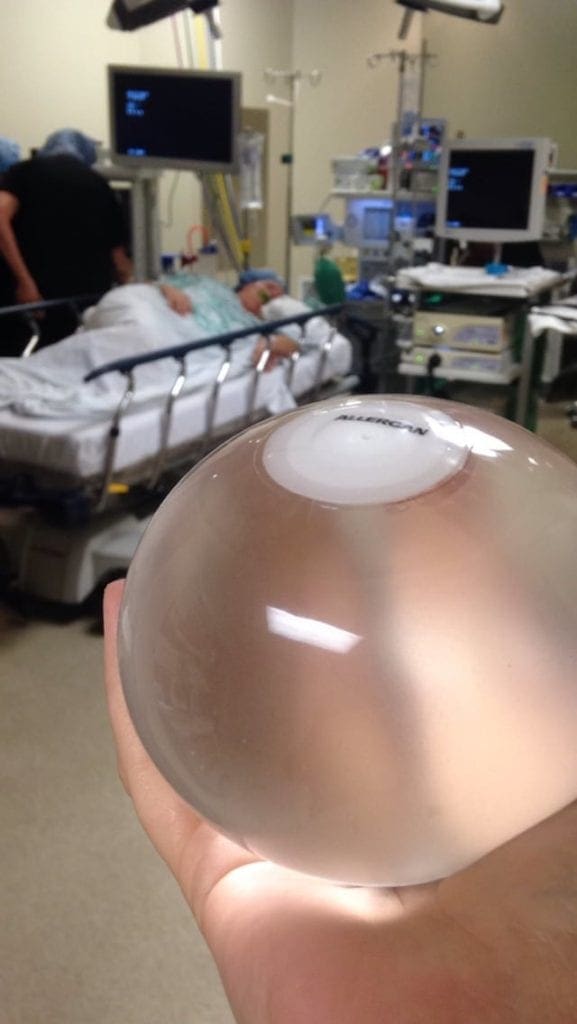
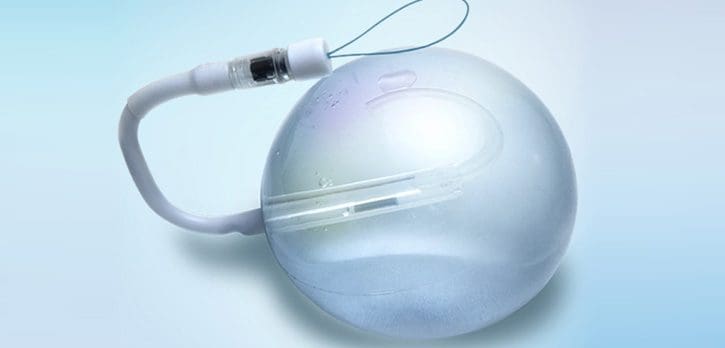
spatz

SURGICAL OPTIONS
NON-SURGICAL OPTIONS
PRICING AND FINANCING